08 Mar What is meant by Off-label

Article by Natalia de la Figuera – Co-Founder and COO of GENESIS Biomed
· Off-label medicines are those prescribed under conditions other than those authorized
· Off-label use is most widespread in oncology, paediatrics, rare diseases, psychiatry, rheumatology and haematology
· There can be different types of off-label prescribing: by indication, by age, by route of administration and by dose
On-label medicines or prescriptions are those medicines that are used in accordance with the characteristics and indications stated in their SmPC (Summary product characteristics). These medicines have been approved by the relevant Medicines Agency and the benefit-risk assessment has been carried out. In other words, under the conditions of use in the SmPC and in the indication reflected, the benefit outweighs the risks, and the regulatory agency has therefore approved their use. But outside the conditions reflected therein, there is not enough information to make decisions.
On the other hand, there are off-label medicines, which are those prescribed under conditions other than those authorized in their SmPC, and therefore not covered by the regulatory agency for commercialization for that purpose, because they have not undergone the necessary benefit-risk assessment.
Although the use of an off-label medicine is NOT regulated by EU legislation, healthcare professionals may consider prescribing an off-label medicine when there is no available treatment for the patient or if an available treatment has not been effective. Off-label prescribing is part of clinical practice and can be based on reliable scientific evidence. Off-label use is more common among certain patient populations, the areas where it is most prevalent are:
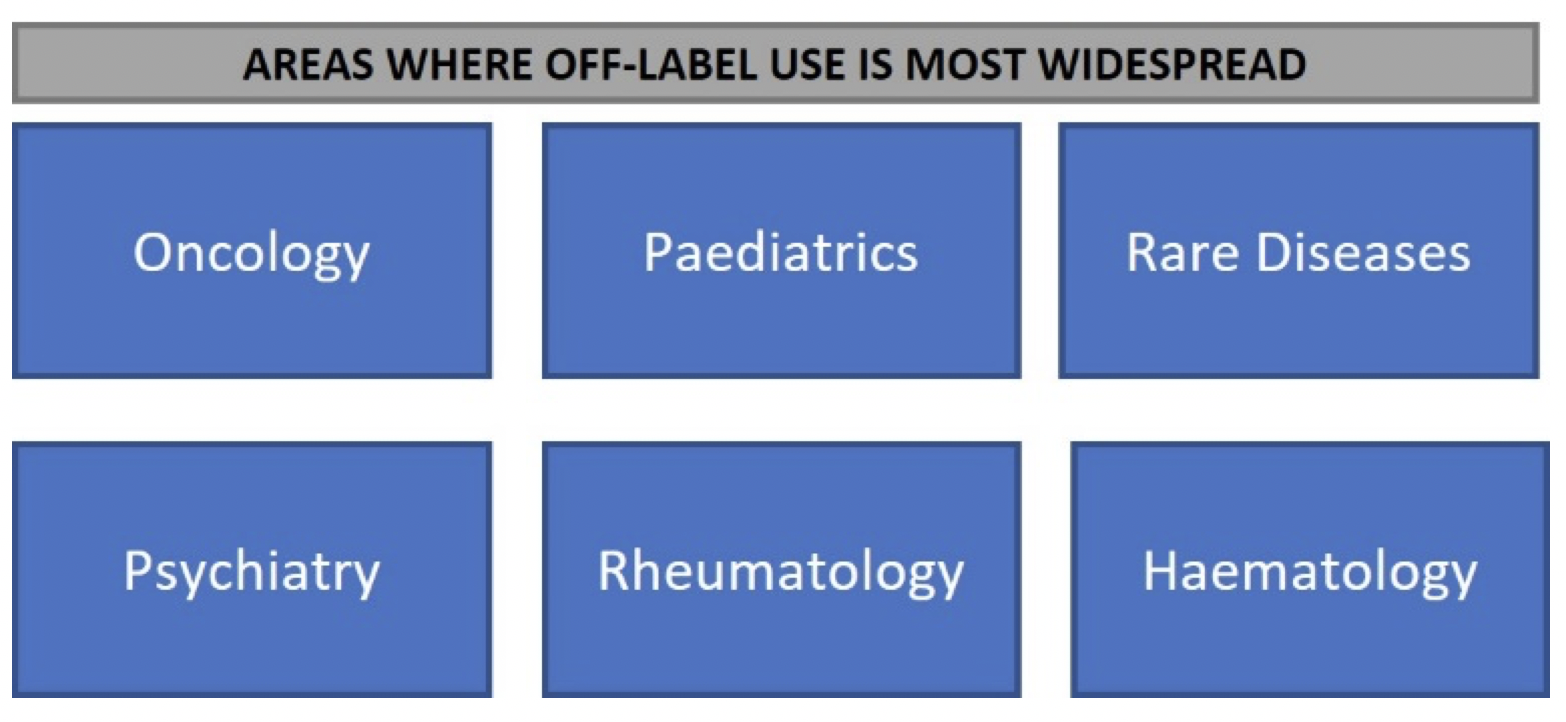
Below are some examples of why off-label medicines use is relevant to some of the medical areas:
Off-label in Oncology:
The use of unapproved drugs is more widespread in oncology than in other medical areas for several reasons, but the main reason is the large number of different types of cancer. Genetic testing shows similarities in mutations in different cancers, which means that a drug against one cancer may be useful in other cancers for which it has not been approved.
Off-label in Paediatrics:
Many paediatric prescriptions are off-label, the reason being that many medicines were not approved for use in paediatric populations and are therefore not authorised for use in children. The most common examples of off-label use in children are medicines that are prescribed at a different dose or frequency than those prescribed for adults. There are also cases where children receive a medicine for a different indication or by an alternative route. The percentage of off-label use varies in paediatrics but ranges from 20-80% of all prescriptions. To regulate its use, on 26 January 2007 the Paediatric Regulation came into effect in the EU to address the problem of off-label use in paediatrics. The regulation aims to improve the health of children in Europe by facilitating the development and availability of medicines for children aged 0-17 years. The regulation requires a company to submit a Paediatric Investigation Plan (PIP) for each medicine it wishes to develop. This ensures that medicines are of high quality, ethically researched and properly authorised, reducing off-label use in this population range.
As seen in the paediatric example, there are different types of off-label prescribing that can clinically occur such as: by indication, by age, by route of administration and by dose. The table below shows the different ways of prescribing medicines and the different uses or modalities of off-label prescribing.
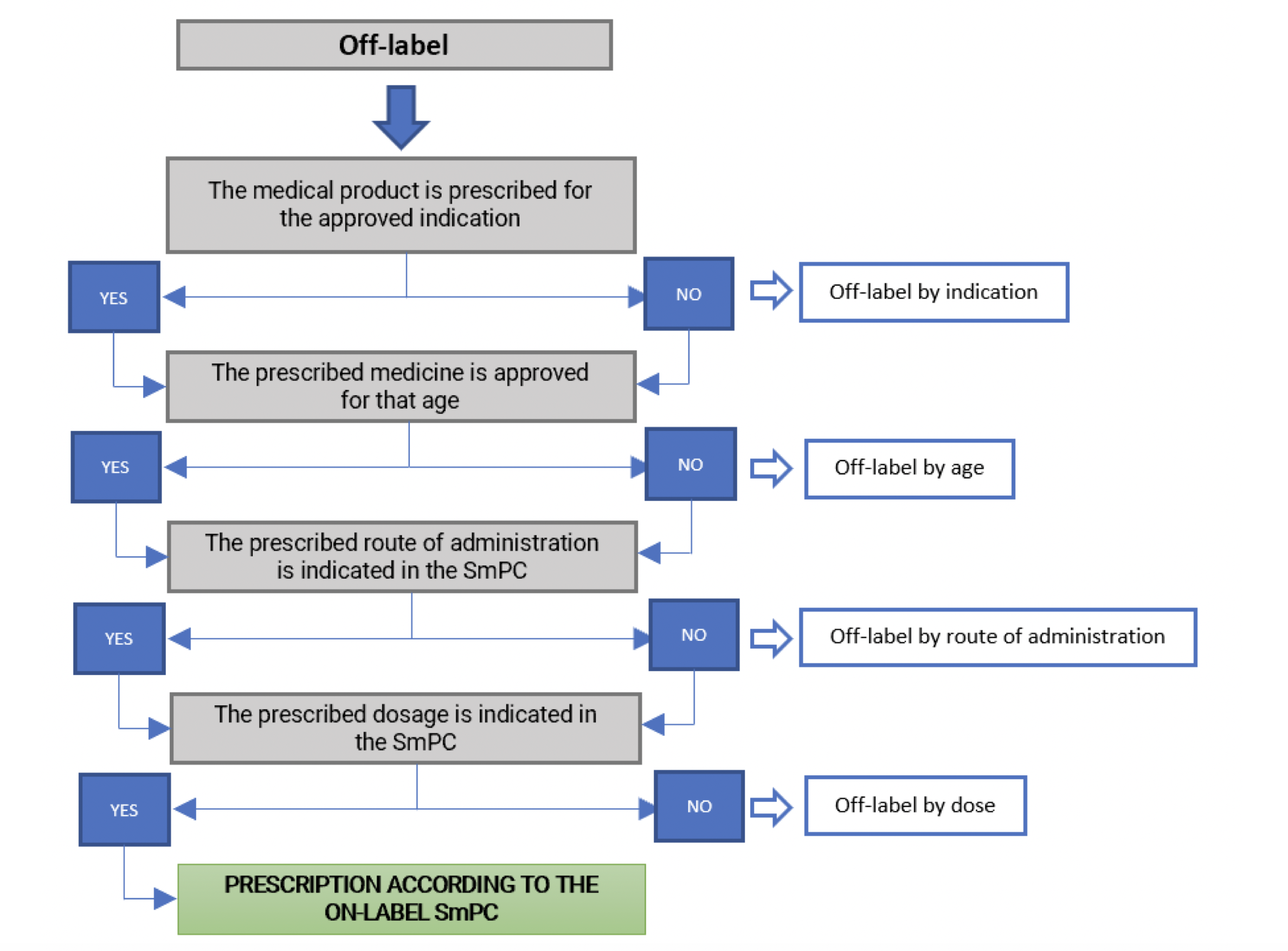
The use of off-label medicines in clinical practice does not require a case-by-case authorisation according to Royal Decree 1015/2009, of 19 June, which regulates the availability of medicines in special situations. However, their use will be exceptional and limited to situations where there is a lack of authorised therapeutic alternatives for a given patient. However, the need for the use of the medicinal product must be adequately justified in the medical record. Inform the patient, in understandable terms, of the nature of the treatment, its importance, implications and risks, and obtain his or her consent. Suspected adverse reactions should also be reported to the Pharmacovigilance system. When prescribing a medicine off-label, healthcare professionals are responsible for any problems arising from the use of the medicine and not the pharmaceutical company.


